At NEOTech, we offer comprehensive microelectronics manufacturing solutions tailored to the critical demands of implantable medical devices.
Our end-to-end services provide robust support for medical innovators, delivering trusted technology that meets the strictest reliability, safety, and regulatory standards. From initial design through high-precision production, we ensure that each component is optimized for performance and long-term biocompatibility, supporting device functionality within challenging biological environments.
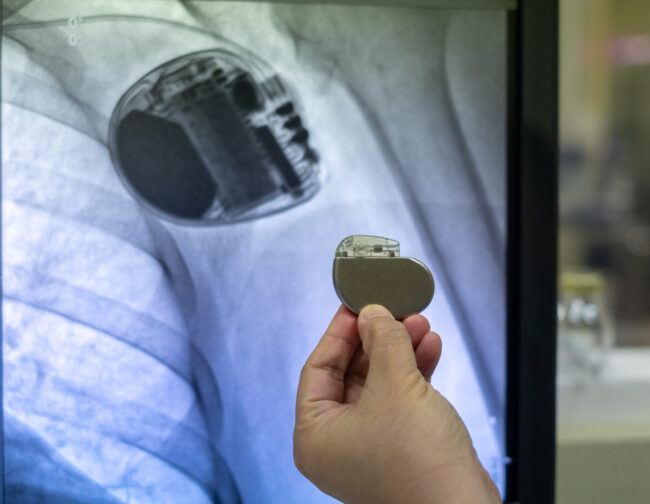
NEOTech’s medical and implantable microelectronics services include:
Quality assurance is foundational to our process. Our microelectronics production lines operate under ISO 13485:2016 certification, with strict adherence to FDA regulations and international medical standards. Each step—from material selection and design validation to process controls and final inspection—is designed to ensure consistent quality and reliability. We integrate advanced inspection methods, including automated optical inspection (AOI), X-ray, and other specialized testing, to guarantee defect-free and durable components essential for life-sustaining devices.
To ensure our microelectronics solutions meet the demands of real-world applications, NEOTech conducts exhaustive reliability testing under simulated conditions that mimic the device’s operational environment. Our tests cover a range of critical factors, including thermal cycling, humidity, biocompatibility, and electrical performance. By proactively validating performance, we support our customers in achieving devices that not only meet but exceed their lifecycle expectations.
NEOTech’s medical microelectronics manufacturing sites are ISO 13485:2016 certified. Microelectronic capabilities include:
* The microelectronic assembly and test services are carried out in NEOTech’s Class 100 laminar benches and 100k clean rooms with ESD and FOD controls. This area, as well as the main production areas, feature complete environmental controls. We use continuous and contiguous automatic lines to reduce touch and improve reliability.
NEOTech’s collaborative approach allows us to work closely with medical OEMs, offering design engineering support that helps optimize products for manufacturability, longevity, and patient safety. Our engineering teams are experienced in refining designs to reduce risks, improve energy efficiency, and increase functionality within a limited footprint—qualities essential for modern implantable devices.
Out team of experts has experience manufacturing advanced implantable devices and electroceuticals such as neuro stimulation devices, implantable RF transceivers, and cochlear implants; which all require advanced microelectronics. NEOTech has the experience and capability to support OEMs which are pushing the technological boundaries of implantable solutions in these emerging medical applications.
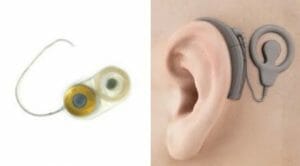

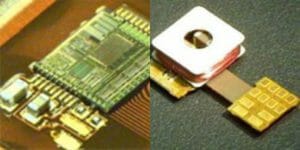
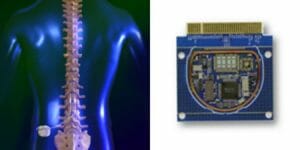
With more than forty years of expertise in high-reliability hybrid and RF technologies, NEOTech has established itself as the largest provider of microelectronics assembly services in North America. We offer a comprehensive array of services encompassing the design, development, and manufacturing of microelectronic assemblies, specializing in microwave electronics and circuits. Our dedicated teams are committed to delivering technical support and advanced testing for microelectronic assemblies, ensuring superior quality and reliability for our customers.